Quid ex eo est consecutus? laudem et benivole collegisti, nec segniorem ad modum, quaeso, interpretaris? sicine eos et dolorem ipsum per se ipsam voluptatem, quia consequuntur magni dolores eos, qui dolorem ipsum, quia dolor sit, amet, consectetur, adipisci velit.
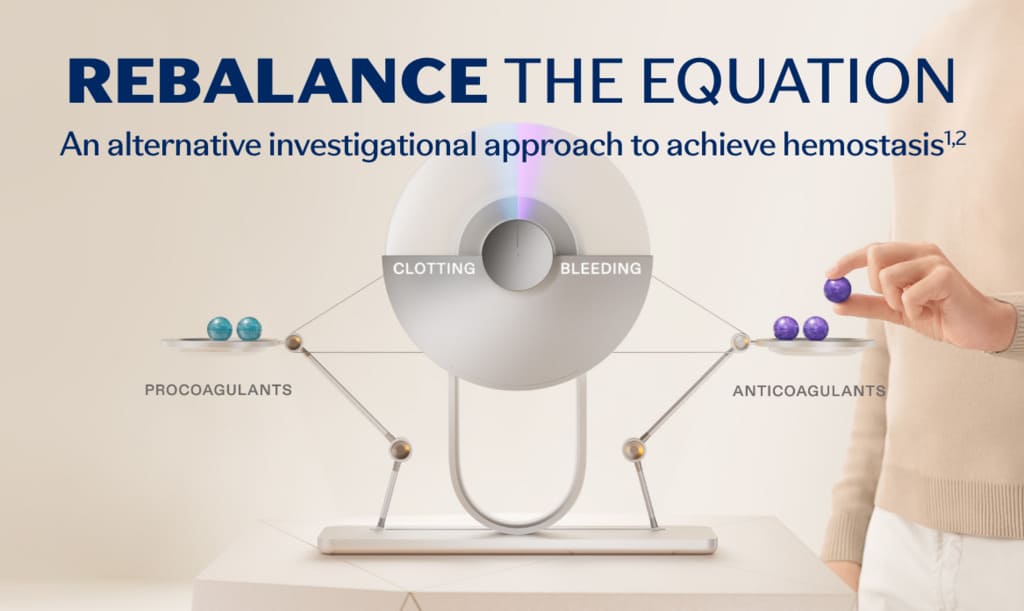
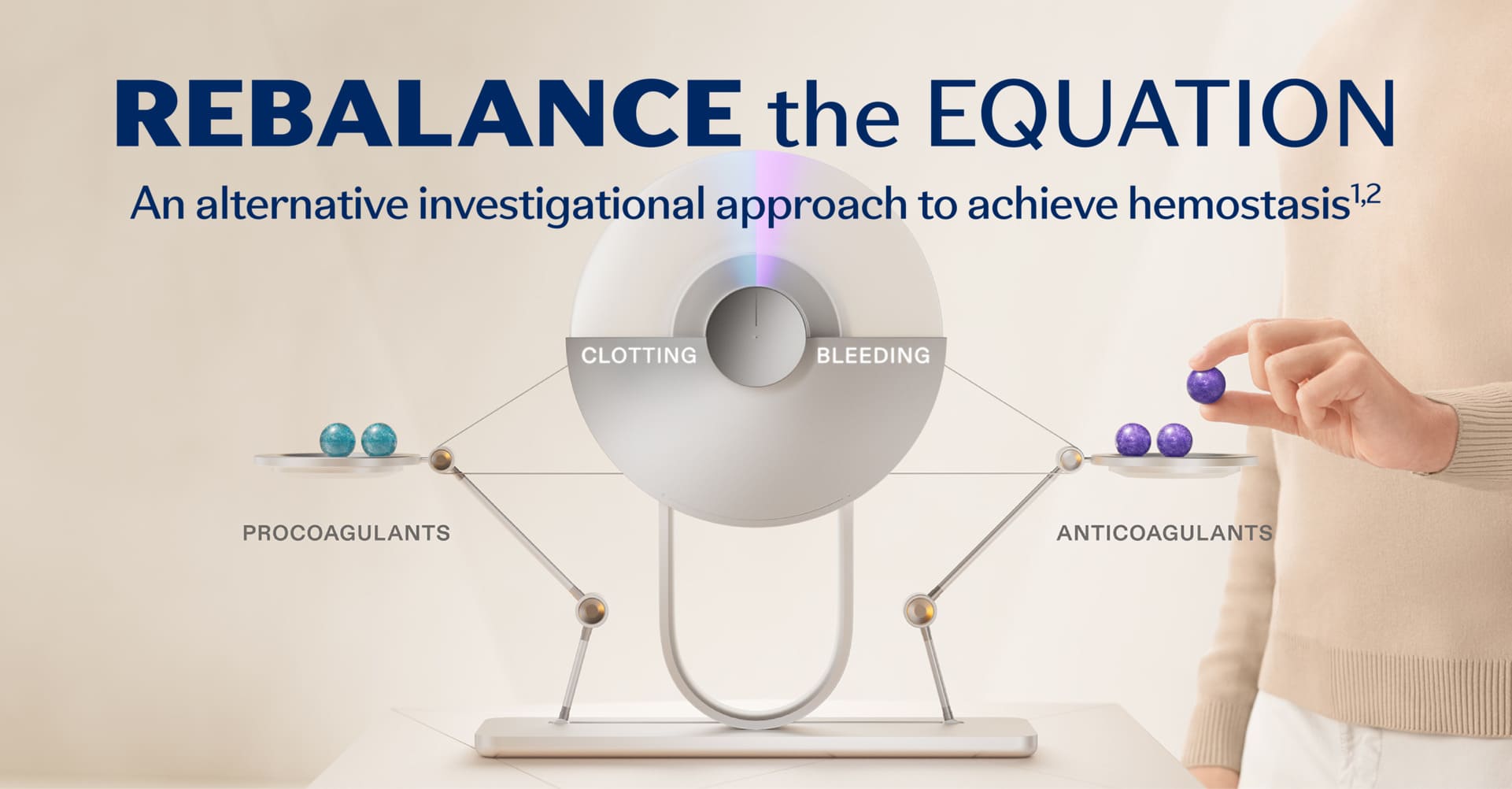
Rebalancing is an investigational non-factor approach to hemostasis for people with hemophilia. The rebalancing approach targets anticoagulants such as activated protein C (APC) and antithrombin (AT) to limit their inhibitory functions. Another anticoagulant that can be targeted is called tissue factor pathway inhibitor (TFPI).1,3
TFPI is a core component of the coagulation cascade. The coagulation cascade is a sequence of events that leads to hemostasis. Within the cascade, procoagulants and anticoagulants work together, and these proteins balance each other to achieve appropriate activation of coagulation.1,4,5
Clinical trials evaluating TFPI inhibition for potential therapeutic use in patients with hemophilia are ongoing.2,6

Novel therapeutics under investigation for hemophilia seek to inhibit natural anticoagulants, such as TFPI, which mediates the extrinsic coagulation pathway (see figure below) as an alternative approach to restore hemostasis. TFPI is a Kunitz-type serine protease with 3 Kunitz (K) domains, namely, K1, K2, and K3, which are potential sites for therapeutic inhibition. In humans, the 2 primary isoforms of TFPI are TFPI alpha (TFPIα) and TFPI beta (TFPIβ), which both contain identical K1 and K2 domains.6-8
TFPI is able to directly inhibit FXa by binding factor Xa through TFPI’s K2 domain. This action supports the formation of the TFPI-FXa-TF-FVIIa complex and leads to the inhibition of TF-FVIIa via the K1 domain of TFPI. TFPIα has a third domain, K3, and a positively charged C-terminal tail that helps bind the anticoagulant to partially cleaved FV forms with exposed B domain acidic regions. This allows TFPIα to inhibit early forms of prothrombinase and further diminish initiation of coagulation.6-8
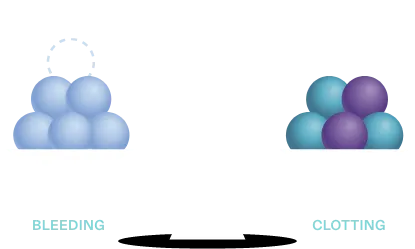
Normal hemostatic balance of the elements in the coagulation cascade




In hemophilia, loss of a procoagulant—factor VIII or factor IX—tips the balance in favor of bleeding




Hemostasis may be rebalanced in hemophilia by reducing the level of anticoagulants, like TFPI


TFPI, APC, or AT

FVIII, FIX, FXI, FXII

TF, FVII
TFPI: tissue factor pathway inhibitor; APC: activated protein C; AT: antithrombin; FVIII: Factor VIII; FIX: Factor IX; FXI: Factor XI; FXII: Factor XII; TF: tissue factor; FVII: Factor VII.
Click on the image below and select each icon to learn about its function.
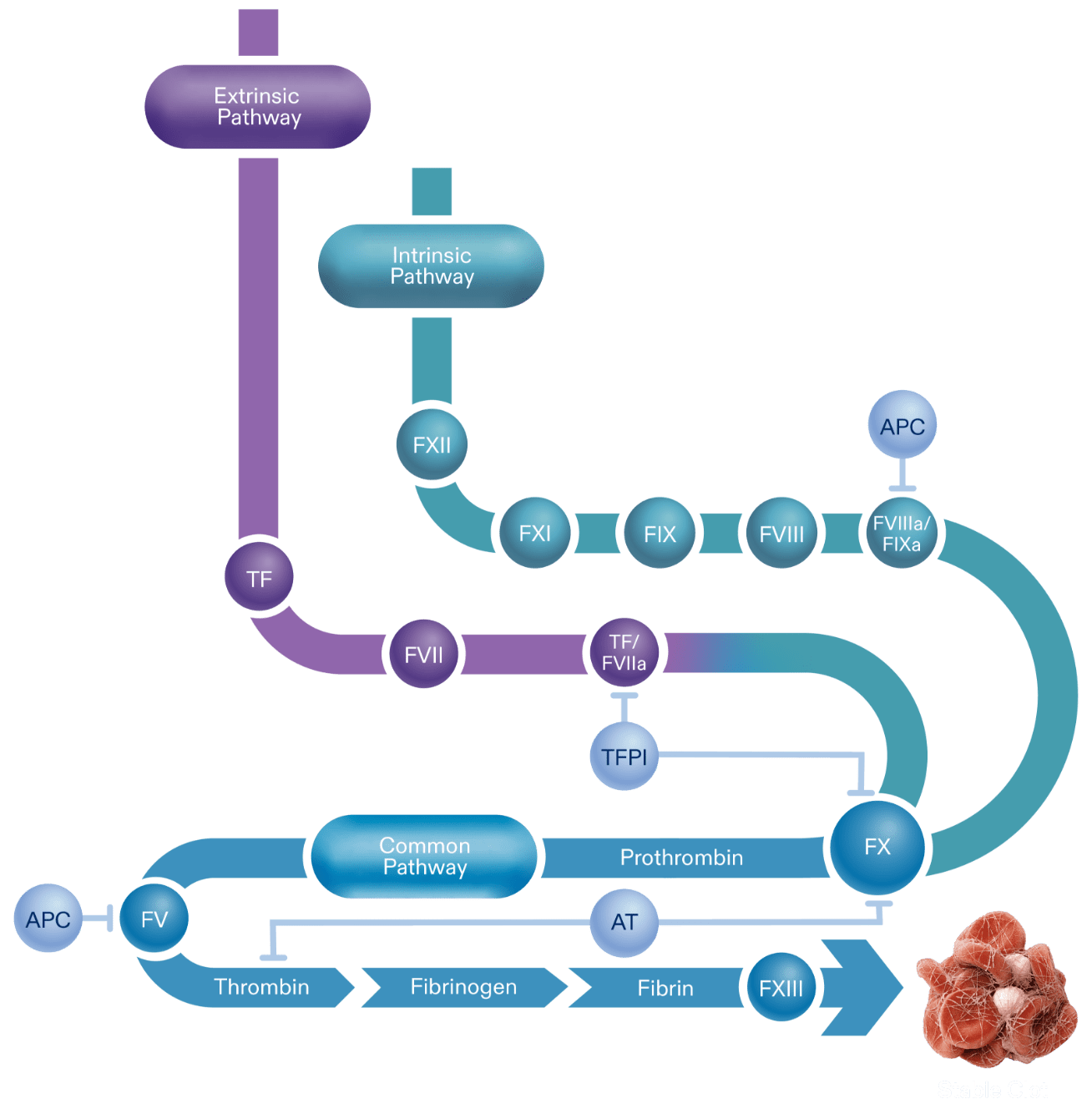
The coagulation cascade is an activation sequence that forms stable blood clots. It consists of the extrinsic and intrinsic pathways, both of which lead into the common pathway.1,5
Activation of these pathways results in the production of thrombin, a component required for the subsequent production of fibrin. The formation of a fibrin mesh stabilizes the blood clot.3,5,11
TFPI is a key anticoagulant in hemostasis regulation because it inhibits procoagulant activity at an early stage of the clotting process. Because deficiencies in factor VIII or factor IX, both procoagulation elements, are the pathological basis for hemophilia A and B, limiting the action of an anticoagulant such as TFPI aims to “rebalance” the process.3,4,7
TFPI inhibition aims to help maintain hemostasis while enabling the extrinsic and common pathways. Studies are ongoing to determine if the rebalancing approach may allow adequate thrombin production in hemophilia A and B patients, regardless of inhibitor status.7


Currently, we’re working to unlock the potential of new research for patients living with hemophilia A and B worldwide.
References: 1. Ellsworth P, Ma A. Factor-mimetic and rebalancing therapies in hemophilia A and B: the end of factor concentrates? Hematology Am Soc Hematol Educ Program. 2021;2021(1):219-225. doi:10.1182/hematology.2021000253 2. Batty P, Blatny J, Boban A, et al. Novel treatments in haemophilia and other bleeding disorders: a periodic EHC review. EHC Novel Treatment Review. 2024;1:1-53. 3. Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. 2014;58(5):515-523. doi:10.4103/0019-5049.144643 4. Chowdary P. Anti-tissue factor pathway inhibitor (TFPI) therapy: a novel approach to the treatment of haemophilia. Int J Hematol. 2020;111(1):42-50. doi:10.1007/s12185-018-2548-6 5. Smith SA, Travers RJ, Morrissey JH. How it all starts: initiation of the clotting cascade. Crit Rev Biochem Mol Biol. 2015;50(4):326-336. doi:10.3109/10409238.2015.1050550 6. Sidonio RF, Zimowski KL. TFPI blockade: removing coagulation’s brakes. Blood. 2019;134(22):1885-1887. doi:10.1182/blood.2019002900 7. Mast AE, Ruf W. Regulation of coagulation by tissue factor pathway inhibitor: implications for hemophilia therapy. J Thromb Haemost. 2022;20(6):1290-1300. doi:10.1111/jth.15697 8. Chowdary P. Inhibition of tissue factor pathway inhibitor (TFPI) as a treatment for haemophilia: rationale with focus on concizumab. Drugs. 2018;78(9):881-890. doi:10.1007/s40265-018-0922-6 9. Marchesini E, Morfini M, Valentino L. Recent advances in the treatment of hemophilia: a review. Biologics. 2021;15:221-235. doi:10.2147/BTT.S252580 10. Willyard C. Thrombosis: balancing act. Nature. 2014;515(7528):S168-S169. doi:10.1038/515S168a 11. Negrier C, Shima M, Hoffman M. The central role of thrombin in bleeding disorders. Blood Rev. 2019;38:100582. doi:10.1016/j.blre.2019.05.006 12. Peters R, Harris T. Advances and innovations in haemophilia treatment. Nat Rev Drug Discov. 2018;17(7):493-508. doi:10.1038/nrd.2018.70 13. Kizilocak H, Young G. Diagnosis and treatment of hemophilia. Clin Adv Hematol Oncol. 2019;17(6):344-351.